Diffuse large B-cell lymphoma (DLBCL) affects some 30,000 patients annually in the United States. While first-line therapies cure approximately two-thirds of patients, those who are not cured or who relapse historically have poor outcomes with standard chemotherapy-based treatment approaches. With findings published in Cancer Research by clinicians at NewYork-Presbyterian/Weill Cornell Medicine, there is reason for optimism for these patients.
In a project encompassing both fundamental research and clinical studies, Sarah Rutherford, MD, a hematologist/oncologist at NewYork-Presbyterian/Weill Medicine, recently co-led a study that demonstrated that a combination of approved chemotherapies, one of which targets the DNA repair-facilitating mechanism, could help treat these persistent cases. The mechanism, which involves the shuttling of messenger RNAs (mRNAs) from the nucleus to the cytoplasm, ultimately facilitates DNA repair in cancer cells that can thereby thwart treatments aimed at damaging their DNA.
Previous research had demonstrated that treatment-resistant DLBCL cells often express high levels of the protein XPO1. In 2019, the U.S. Food and Drug Administration approved a new oral drug, selinexor, that was designed to target XPO1 and inhibit its activity. The drug hinders the growth of lymphoma cells expressing high levels of the protein as demonstrated in the SADAL trial, a multinational, multicenter phase 2 trial. The trial showed that selinexor not only produced an enduring response and improved survival in patients with relapsed or refractory disease, but the drug’s side effects also were expected and manageable. However, selinexor’s use as a single agent has been modest.
“Selinexor is effective by itself, but we expect it to be more effective in combination with other therapies,” says Dr. Rutherford, whose research has sought ways to improve the drug’s efficacy.
Early Due Diligence for Selinexor
In 2015, Peter Martin, MD, Chief of the Lymphoma Program at Weill Cornell Medicine, served as an investigator in a multicenter phase 1 dose-escalation study to assess the effects of oral selinexor in patients with advanced hematologic malignancies. The study published in 2017 found that selinexor was a promising treatment strategy for several subtypes of relapsed/refractory non-Hodgkin lymphoma.
In collaboration with investigators at the National Cancer Center Singapore, Dr. Martin played a key role in developing and supervising a phase 1 study to determine the safety and maximum tolerated dose of selinexor in combination with high-dose dexamethasone and standard dose ifosfamide, carboplatin, etoposide (ICE) chemotherapy in patients with relapsed or refractory T-cell lymphoma. The researchers found that the maximum tolerated dose of selinexor that could be used with this combination in a 21-day cycle was 40 mg on days 3, 5 and 7. In the 10 patients evaluated, overall and complete response rates were 91 percent and 82 percent, respectively, and although there were concerns about the side effect profile, the investigators determined the approach warranted further study.
At the same time, a number of other Weill Cornell Medicine hematologists/oncologists had been seeking to understand more about how selinexor works. Its target, XPO1, transports hundreds of proteins, and certain RNAs, out of the cell nucleus, primarily to separate the pool of proteins that should not be present in the nucleus, such as ribosomal proteins. However, their investigations found that some of these XPO1-exported proteins are also bound to mRNA molecules; thus, these mRNAs are exported out of the cell nucleus into the cytoplasm where they can be translated into proteins. This new mechanism indicates that the quantity and activity of XPO1 in a cell can therefore affect the expression levels of numerous genes.
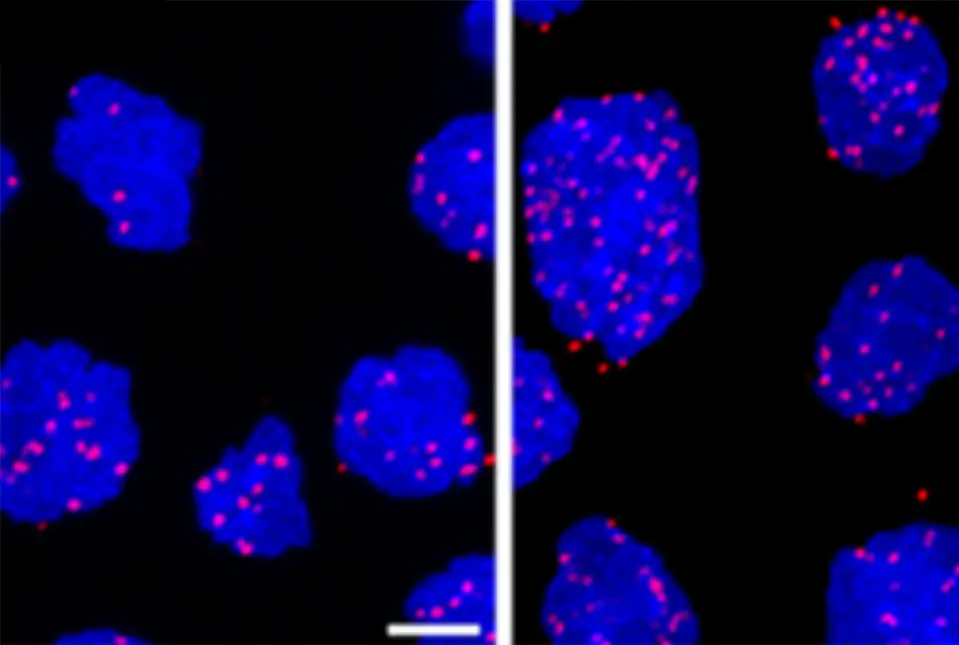
Diffuse large B cell lymphoma cells that are untreated (left panel) and treated with a DNA damaging agent (right panel). The pink dots indicate XPO-1 protein interacting with a protein that binds mRNAs and chaperones them from the nucleus.
“We found that it’s not just regulating a few proteins, it’s coordinating these big programs, allowing cells to rapidly adjust their proteome and survive different kinds of stress that cancer cells encounter all the time,” says senior author Leandro Cerchietti, MD, a physician-scientist at Weill Cornell Medicine.
Previously, Dr. Cerchietti and his team, including Rossella Marullo, MD, PhD, an instructor in medicine at Weill Cornell Medicine and co-lead author of the study, had extracted treatment resistant DLBCL cells from patients and grafted them into patient-derived xenograft models. They found that higher levels of XPO1 ultimately increase the expression of genes that protect cells against death from DNA damage. Inhibiting XPO1 in those models with selinexor increased the lymphomas’ sensitivity to DNA-damaging chemotherapies and immune-based treatments.
“Selinexor is a first-in-class selective inhibitor of nuclear export, and it works by inhibiting the transport of various different materials, including mRNAs and proteins, in and out of the nucleus of a cell,” explains Dr. Rutherford. “It has been shown preclinically to be effective, particularly in interfering with the shuttling back and forth of these different molecules. In particular, some are very important in the pathogenesis of diffuse large B-cell lymphoma – C-MYC, BCL-2, and BCL-6. Dr. Cerchietti and his team were able to develop a preclinical model of lymphomas with rearrangements involving those three genes – also called triple-hit lymphoma.”
Taking Investigations to the Next Level
“We were excited about Dr. Cerchietti’s research and thought selinexor would likely synergize with other chemotherapies,” says Dr. Rutherford. To that end, she initiated a phase 1 clinical dose-finding trial in 2015, aiming to determine whether such a combination would be safe, and if so, at what doses. The trial, which enrolled 22 patients primarily with treatment-resistant DLBCL, showed that the combined regimen is not only safe but appears to work.
“We combined selinexor with a standard chemotherapy regimen R-ICE – rituximab, ifosfamide, carboplatin, and etoposide,” says Dr. Rutherford. “Ultimately, we found the 40 mg dose of selinexor in combination with the R-ICE treatment to be a tolerated regimen with some very good results. We had an overall response rate of 71 percent in the patients who received selinexor with R-ICE and a number of them were able to go on to autologous stem cell transplant or CAR T-cell therapy. Many of them are still in remission years after the initial treatment.”
Ultimately, we found the 40 mg dose of selinexor in combination with the R-ICE treatment to be a tolerated regimen with some very good results. We had an overall response rate of 71 percent in the patients who received selinexor with R-ICE and a number of them were able to go on to autologous stem cell transplant or CAR T-cell therapy.
— Dr. Sarah Rutherford
“Traditionally in chemotherapy-based clinical trials it was thought that higher doses were more effective,” adds Dr. Rutherford. “But with targeted therapies, often you don’t need as high a dose. You just need some amount of it to help inhibit, and in this case, inhibit the nuclear pore activity. If a person can tolerate a lower dose for longer, that’s going to be better than having to interrupt the therapy because the patient is having side effects at the higher doses. It’s a different treatment strategy.”
Because every cell in the body expresses XPO1, the new results are likely to have broader applications. “There are other tumors in which XPO1 is overexpressed, so it’s really a nice backbone to build on,” says Dr. Cerchietti.
Emerging New Therapies
Dr. Rutherford plans to continue testing and refining the new regimens in follow-up trials. “It has been an exceptional time in this disease over the last four years or so, where we now have many more therapies than we did when we first started the trial,” she says. “CAR T-cell therapies target CD19, a protein on the surface of lymphoma cells. We have other therapies that target CD19, including tafasitamab, a monoclonal antibody that’s given in combination with lenalidomide, and loncastuximab tesirine, an antibody drug conjugate. Polatuzumab vedotin, which targets the protein CD79b on the surface of the lymphoma cells, and more recently, Pola-R-CHP, have been approved in the first-line setting and are considered breakthrough therapies, adding treatment options to R-CHOP, which had been the standard of care for diffuse large B-cell lymphoma.”
“I couldn't have imagined that so much progress would have been made in this field since I joined NewYork-Presbyterian/Weill Cornell Medicine 12 years ago,” adds Dr. Rutherford. “We have such good relationships between the clinical and research groups and are able to design clinical trials like this one based on preclinical work. We’ve contributed to the literature and to the knowledge that we can combine this new novel selinexor with chemotherapy, and I'm excited about continuing research with that type of drug in combination with other agents for DLBCL and other cancers.”
Many NewYork-Presbyterian/



